Abstract
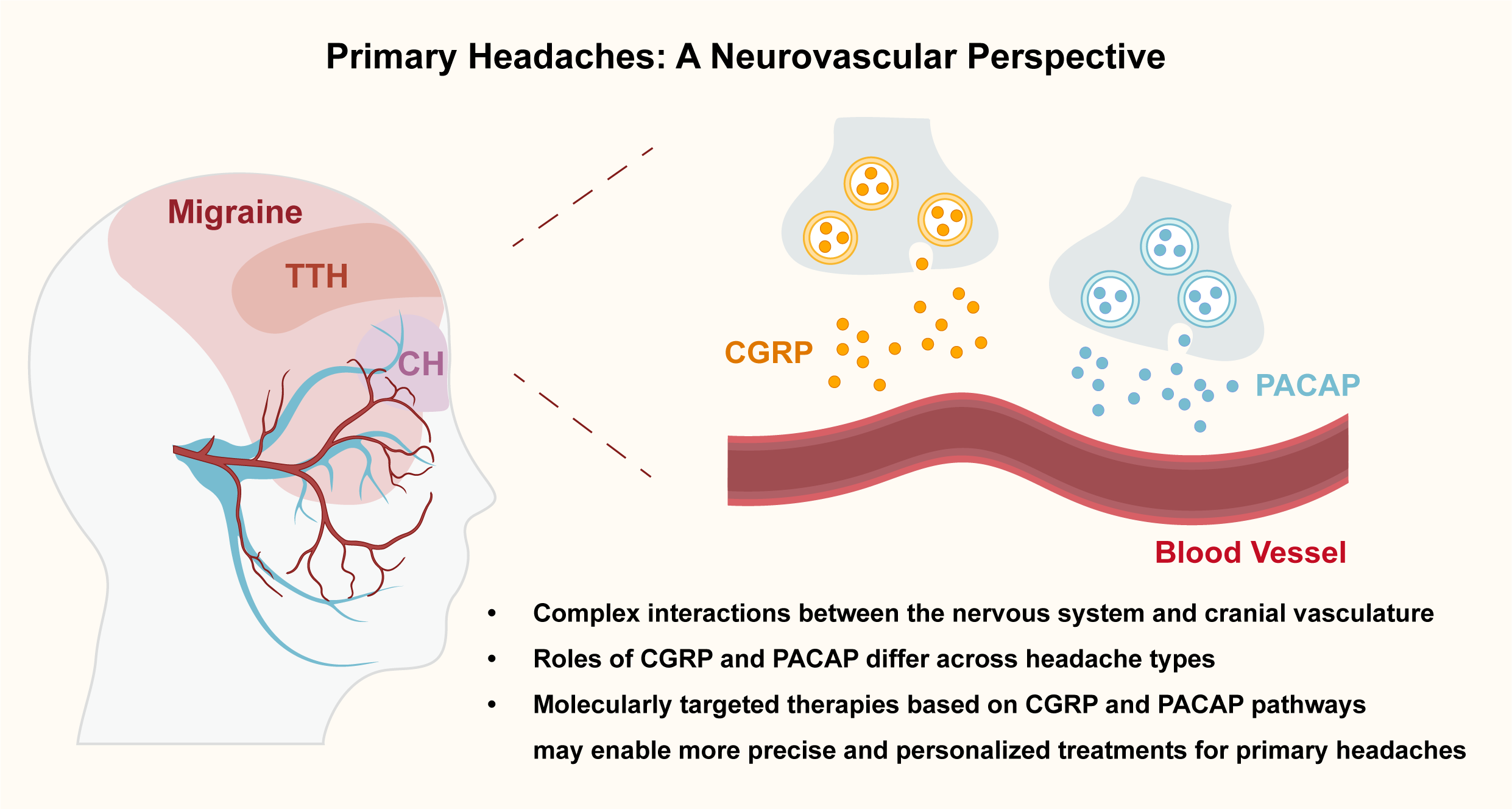
Primary headaches such as migraine, cluster headache (CH), and tension-type headache (TTH) are highly prevalent neurological disorders with complex and heterogeneous pathophysiology. Traditionally attributed to either vascular or neuronal origins, current evidence supports a neurovascular model involving dynamic interactions between the nervous system and cranial. This Perspective examines the role of two key neuropeptides – calcitonin gene-related peptide (CGRP) and pituitary adenylate cyclase-activating polypeptide (PACAP) – in the generation of primary headaches. CGRP is a validated target in migraine, but its clinical utility is limited by biomarker instability and a significant proportion of non-responders. PACAP is emerging as a complementary target, though its receptor mechanisms remain incompletely understood. While neither CGRP nor PACAP appear to play a major role in TTH, their contribution to migraine and CH highlights the need for precision approaches based on molecular endophenotypes. Understanding these mechanisms may inform the development of more effective, personalized headache treatments.
Introduction
Primary headaches such as migraine, cluster headache (CH), and tension-type headache (TTH) represent a significant burden for patients and healthcare systems worldwide [1] . Despite advances in symptomatic and preventive treatments, many aspects of their underlying mechanisms remain incompletely understood, contributing to the variability in therapeutic response and the lack of predictive biomarkers. Traditionally, primary headaches were conceptualized as either purely vascular or purely neuronal disorders [2] . However, accumulating evidence now supports a more integrated neurovascular perspective, in which complex and dynamic interactions between the nervous system and cranial vasculature contribute to headache pathophysiology [3] . Among the most studied mediators in this context are the trigeminovascular neuropeptides – calcitonin gene-related peptide (CGRP) and pituitary adenylate cyclase-activating polypeptide (PACAP). In addition to CGRP and PACAP, several other neuropeptides have been implicated in headache disorders, including substance P, neurokinin A, nerve growth factor, beta-endorphin, and neuron-specific enolase, each contributing through diverse pathways. These peptides have been shown to trigger migraine attacks when administered experimentally and have become key targets in the development of new preventive therapies [4,5] . However, the mechanistic roles of CGRP and PACAP differ across headache types, and their clinical utility as biomarkers or universal targets remains limited by biological complexity and patient heterogeneity. This mini-review discusses current knowledge and open questions regarding the mechanisms of primary headaches, with a focus on the vascular vs. neuronal debate, the central yet partial role of CGRP, and the emerging but still puzzling contribution of PACAP. Special attention is given to how these mechanisms differ across migraine, cluster headache, and tension-type headache, and what these insights mean for the future of targeted therapies (Figure 1).
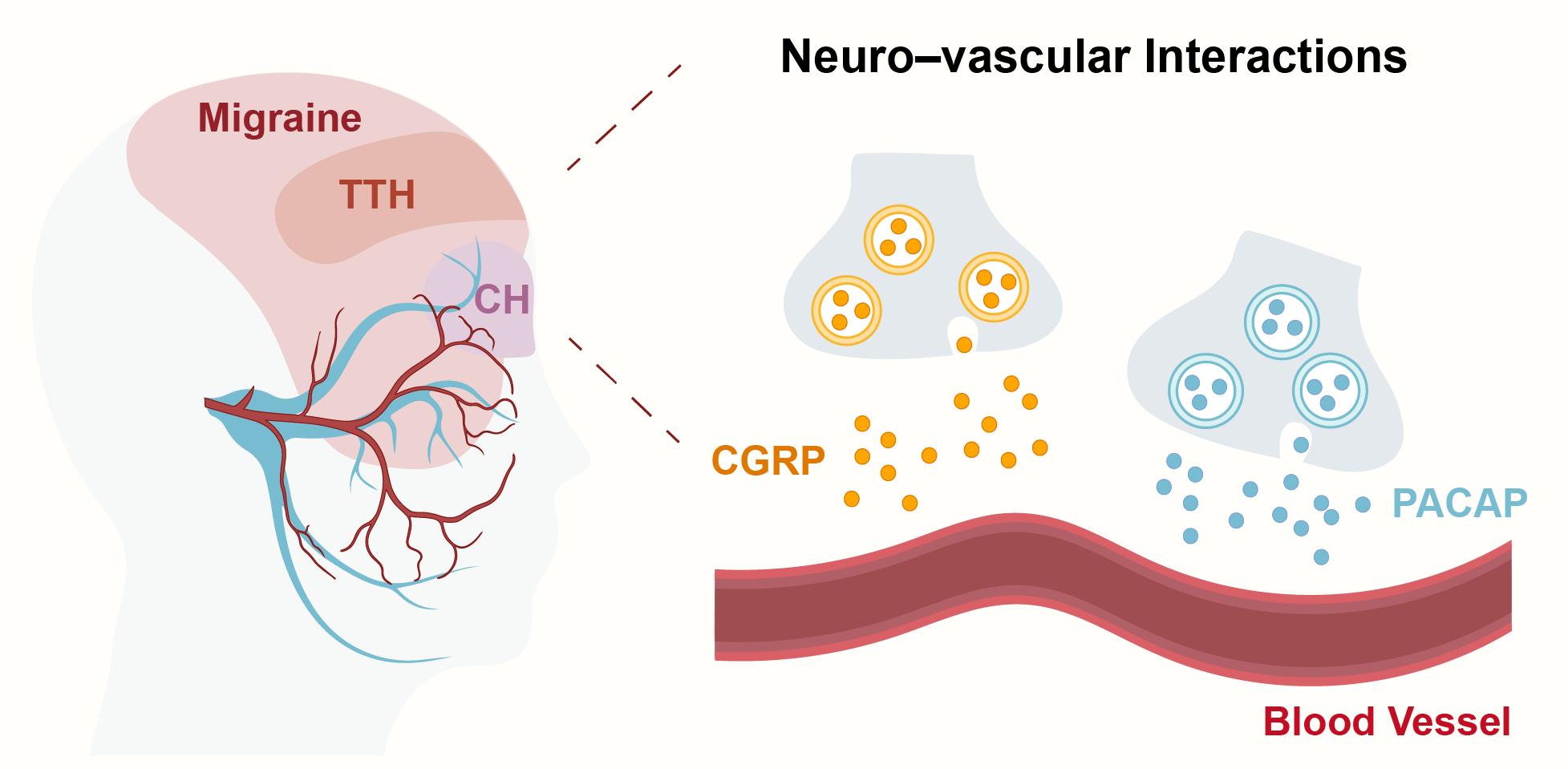
Figure 1. Neurovascular Mechanisms in Primary Headaches at the Trigeminovascular Interface. CH: cluster headache. CGRP: calcitonin gene-related peptide. PACAP: pituitary adenylate cyclase-activating polypeptide. TTH: tension-type headache.
Neuronal vs. vascular origin
The longstanding debate over whether headaches – particularly migraine – arise from a primary vascular disturbance or a neuronal dysfunction remains unresolved [6] . Historically, the vascular theory prevailed, grounded in the observation that cerebral vessels are pain-sensitive, and that vasoconstrictors such as ergotamine could alleviate attacks [7,8] . However, accumulating neuroimaging and neurophysiological data have shifted the focus toward a neurogenic origin [9] . Studies revealing early activation of brainstem and hypothalamic nuclei prior to pain onset suggest that vasodilation may be a secondary phenomenon, rather than the initiating event. Rather than endorsing a binary view, modern understanding favors a neurovascular model, recognizing that headache disorders likely emerge from complex and bidirectional interactions between neural and vascular components [10] . A compelling example of this complexity comes from human provocation studies with vasoactive intestinal peptide (VIP). A short 20-minute infusion of VIP failed to induce migraine in individuals with a history of migraine without aura [11] , yet when infused over two hours, VIP provoked migraine attacks in over 70% of participants [12] . These findings indicate that headache induction is not solely a function of vasodilation, but depends on the duration, context, and downstream molecular consequences of vascular activation. Sustained vascular changes may interact with perivascular peptidergic fibers, particularly sensory neurons of the trigeminovascular system, to create a permissive environment for nociceptive activation. This neurovascular cross-talk likely involves reciprocal signaling between vascular smooth muscle cells, endothelial cells, immune components (such as mast cells), and sensory afferents, all of which can dynamically modulate the threshold for pain initiation. Central to this process is the release of key neuropeptides like CGRP and substance P [13] . These peptides promote vasodilation, mast cell degranulation, and sterile neurogenic inflammation in the meninges, events that can serve as both triggers and amplifiers of pain in genetically susceptible individuals. Advanced neuroimaging studies highlight the role of central brain networks, particularly within the hypothalamus and brainstem, as potential generators of migraine attacks. These regions may contribute to the disruption of homeostatic regulation, thereby increasing susceptibility of the peripheral trigeminovascular system to activation [14,15] . The heterogeneity in patient presentation and therapeutic response suggests that no single pathway explains all cases. Instead, the current consensus embraces the idea of dynamic and individualized neurovascular interactions, which may differ across clinical phenotypes and genetic backgrounds. Clinical heterogeneity across primary headache subtypes may be explained, at least in part, by the relative contribution of neuronal and vascular mechanisms within the neurovascular model. Cluster headache is characterized by profound cranial autonomic features and hypothalamic involvement, suggesting a strong vascular-autonomic component that may act as a driver rather than a consequence of pain [16] . Tension-type headache, by comparison, lacks consistent evidence of either vasodilation or neuropeptide involvement, and may instead reflect dysfunctional central pain processing with minimal peripheral input [17] . These distinctions suggest that while neurovascular interactions are a common framework, the dominance of one mechanism over the other may vary by subtype and even within individual patients over time. Acknowledging this variability is key to developing more personalized and mechanism-based treatments.
CGRP: key player, but not the whole story
CGRP is widely recognized as a central mediator in migraine pathophysiology [18] . Elevated levels of CGRP have been observed during spontaneous and experimentally induced migraine attacks, and the efficacy of CGRP-targeted therapies has validated its mechanistic relevance. Currently approved CGRP-targeted therapies include monoclonal antibodies against the ligand (eptinezumab, fremanezumab, galcanezumab) or the receptor (erenumab), as well as small-molecule receptor antagonists (rimegepant, ubrogepant, and atogepant). These agents have shown moderate to high efficacy in reducing monthly migraine days and acute attack severity in randomized controlled trials [19] . However, despite its biological plausibility, CGRP has not yet emerged as a reliable clinical biomarker. Its concentration fluctuates considerably depending on the biological matrix analyzed (e.g., jugular vs. peripheral blood, plasma vs. serum), the migraine phase (ictal vs. interictal), and the analytical methodology employed (e.g., RIA, ELISA, or EIA) [20] . In addition to this variability, CGRP is highly labile, with a short half-life of 7-9 minutes, and is sensitive to pre-analytical handling and degradation during sample processing [21] . To overcome these limitations, recent studies have explored alternative biological fluids such as saliva and tear fluid, which are more directly innervated by the trigeminal system and exhibit higher CGRP concentrations. These methods, which allow for non-invasive and repeated self-sampling, have suggested the existence of CGRP-dependent and CGRP-independent migraine attacks, supporting the existence of biologically distinct migraine endophenotypes [22,23] . Nonetheless, these promising findings remain preliminary and require confirmation through larger, well-controlled, and standardized studies. Another limitation of CGRP-targeted therapy is the presence of non-responders, a phenomenon consistently observed in real-world settings. Approximately 30-40% of patients with migraine – particularly those with chronic forms – do not experience meaningful benefit from monoclonal antibodies targeting the CGRP ligand or receptor [24] . Notably, some patients remain unresponsive even after switching between therapeutic targets (ligand vs. receptor), suggesting that CGRP is not the sole driver of migraine pain. Other neuropeptides, such as PACAP, substance P, and neurokinin A, may contribute independently or synergistically to headache generation. Several mechanisms have been proposed to explain the substantial proportion of patients who do not respond to CGRP-targeted therapies [25] . One possibility involves genetic polymorphisms affecting CGRP receptors or downstream signaling pathways, which could alter drug binding or intracellular responses. Receptor desensitization or internalization, particularly with prolonged exposure to monoclonal antibodies, may also diminish clinical efficacy over time. Alternatively, non-CGRP pathways may become upregulated in non-responders, creating redundant or compensatory circuits that bypass CGRP inhibition. For instance, PACAP expression may be increased in some patients as an adaptive mechanism, contributing to persistent headache despite CGRP blockade [26] . Other neuropeptides such as substance P and neurokinin A may similarly sustain nociceptive signaling. In CH, CGRP’s role appears more limited and heterogeneous. While galcanezumab demonstrated efficacy in reducing attack frequency in episodic CH, it failed to show benefit in the chronic subtype, as did fremanezumab in both episodic and chronic forms [27,28] . These findings point to a restricted or context-specific involvement of CGRP in CH, possibly limited to certain phases or subgroups of patients [29] . Given the strong involvement of hypothalamic and autonomic circuits in CH pathophysiology, CGRP may function more as a modulator than a primary initiator of attacks. In TTH, current evidence does not support a relevant role for CGRP. Studies measuring its concentration in plasma and cerebrospinal fluid have found no significant differences between patients with TTH and healthy controls [30,31] . Accordingly, CGRP-targeted therapies have not been investigated in this population and are unlikely to be of therapeutic value.
PACAP: a promising but puzzling target
Currently approved CGRP-targeted therapies are ineffective in approximately 40% of migraine patients [24] , leading to the hypothesis that alternative mechanisms such as the PACAP pathway may contribute to disease pathophysiology in this subgroup. PACAP has emerged as a promising, though complex, therapeutic target across primary headache disorders. Sharing 68% sequence homology with VIP, PACAP binds to a family of receptors – PAC1, VPAC1, and VPAC2 – which are widely expressed in migraine-relevant anatomical sites such as the trigeminal ganglia, sphenopalatine ganglia, and cranial blood vessels [32-34] . A recent proof-of-concept trial using a neutralizing antibody against circulating PACAP demonstrated a reduction in monthly migraine days in patients with prior failure to at least two preventive therapies [35] . Although the trial was limited by its short duration (4 weeks) and small sample size, it offers an encouraging signal for the development of PACAP-targeted strategies as an alternative to CGRP-directed therapies. However, the precise receptor mechanisms underlying PACAP-induced headache remain unclear. Early efforts focused on the PAC1 receptor, given its high affinity for PACAP, but a phase II trial of a monoclonal antibody against PAC1 failed to show superiority over placebo in migraine prevention [36] . As a result, researchers have begun to explore the potential role of VPAC1 and VPAC2 receptors, which bind PACAP with equal affinity and may contribute to migraine induction [37] . Furthermore, mast cells express MRGPRX2, a receptor implicated in PACAP-induced migraine-like behavior in animal models [38] . Other targets such as GPR55, initially described as a cannabinoid-related receptor, have also been implicated in pain signaling and sensitization, and may contribute to the broader PACAP response [39] . The receptor pathways responsible for PACAP-induced migraine remain incompletely elucidated, and PACAP’s role across the spectrum of primary headaches is still being defined. Systematic comparisons across PAC1, VPAC1/2, and MRGPRX2 receptor pathways remain limited by the scarcity of receptor-selective ligands and the lack of validated antibodies for human tissue studies. As a result, mechanistic insights are largely derived from preclinical models and remain difficult to translate into distinct therapeutic strategies. In TTH, available data are scarce and inconclusive. One study found no significant differences in interictal plasma PACAP levels between patients with TTH and healthy controls, suggesting a negligible role for PACAP in this condition [40] . In contrast, CH appears to involve PACAP more directly. Plasma PACAP levels have been found to be reduced during the inter-bout period in episodic CH but elevated during active attacks [41] . A double-blind, placebo-controlled crossover study demonstrated that 20-minute PACAP infusions triggered cluster-like attacks in approximately 50% of patients with active episodic or chronic CH, while patients in remission were unaffected [42] . These PACAP-provoked attacks occurred independently of changes in CGRP, VIP, or mast cell activation markers such as histamine and tryptase [43,44] , implying a distinct and non-redundant mechanism within the pathophysiological framework. PACAP may act as a shared, but variably expressed and weighted, mediator across primary headache types. While its role in TTH appears minimal, its pathophysiological and clinical relevance in migraine and CH supports further investigation into PACAP-targeted therapies.
Conclusions
Understanding the pathophysiological mechanisms of primary headaches remains a critical challenge. While historical theories emphasized either vascular or neuronal origins, current evidence supports an integrated neurovascular model, in which the dynamic interplay between neural and vascular systems contributes to the generation and modulation of headache pain. This complexity is exemplified by neuropeptides such as VIP, CGRP, and PACAP, which exert both vasoactive and neuronal effects [45] . CGRP is a therapeutic target in migraine, yet its clinical utility is limited by lack of biomarker reliability and a substantial proportion of non-responders. PACAP represents a newer target, with preliminary evidence supporting its role in both migraine and cluster headache, but not in tension-type headache. The incomplete understanding of PACAP’s receptor mechanisms highlights the need for further mechanistic and translational research. Future research should aim to better characterize biologically defined subtypes, identify predictive biomarkers, and develop targeted therapies that address the specific mechanisms active in individual patients. As the field advances, future therapeutic strategies may include dual-target inhibitors that simultaneously modulate CGRP and PACAP pathways, as well as personalized approaches based on biomarker profiles or headache phenotypes. While comparative efficacy and side effect profiles across existing treatments remain insufficiently characterized, ongoing trials and expert consensus initiatives are expected to inform the clinical positioning of neuropeptide-targeted therapies [45] . Continued mechanistic research will be essential to guide rational combination or sequential therapies in patients who remain refractory to current options. Integrating insights from neuroimaging, molecular biology, and human provocation models will be key to advancing precision medicine in the field of headache disorders.
Declarations
Author contributions
LP wrote the first draft of the manuscript. WW and YL revised the manuscript and contributed to its final writing. YL prepared the figure and the graphical abstract.
Acknowledgements
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding information
Not applicable.
Competing Interests
The authors declare that they have no existing or potential commercial or financial relationships that could create a conflict of interest at the time of conducting this study.
Data Availability
Not applicable.
References
[1] Waliszewska-Prosół M, Montisano DA, Antolak M, Bighiani F, Cammarota F, Cetta I, et al. (2024). The impact of primary headaches on disability outcomes: a literature review and meta-analysis to inform future iterations of the Global Burden of Disease study. J Headache Pain. 2024;25(1):27. https://doi.org/10.1186/s10194-024-01735-0.
[2] Moskowitz MA. (2007). Pathophysiology of headache—past and present. Headache. 2007;47 Suppl 1:S58-63. https://doi.org/10.1111/j.1526-4610.2007.00678.x.
[3] Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. (2019). Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol. 2019;18(8):795-804. https://doi.org/10.1016/S1474-4422(19)30185-1.
[4] Guo S, Jansen-Olesen I, Olesen J, Christensen SL. (2023). Role of PACAP in migraine: An alternative to CGRP? Neurobiol Dis. 2023;176:105946. https://doi.org/10.1016/j.nbd.2022.105946.
[5] Kuburas A, Russo AF. (2023). Shared and independent roles of CGRP and PACAP in migraine pathophysiology. J Headache Pain. 2023;24(1):34. https://doi.org/10.1186/s10194-023-01569-2.
[6] Jacobs B, Dussor G. (2016). Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience. 2016;338:130-44. https://doi.org/10.1016/j.neuroscience.2016.06.012.
[7] Ray B, Wolff H. (1940). Experimental studies on headache. Pain sensitive structures of the head and their significance in headache. Arch Surg. 1940;41(4):813-56. https://doi.org/10.1001/archsurg.1940.01210040002001.
[8] Tunis MM, Wolff HG. (1953). Studies on headache; long-term observations of the reactivity of the cranial arteries in subjects with vascular headache of the migraine type. AMA Arch Neurol Psychiatry. 1953;70(5):551-7. https://doi.org/10.1001/archneurpsyc.1953.02320350003001.
[9] Goadsby PJ. (2009). The vascular theory of migraine—a great story wrecked by the facts. Brain. 2009;132(Pt 1):6-7. https://doi.org/10.1093/brain/awn321.
[10] Ashina M. (2020). Migraine. N Engl J Med. 2020;383(19):1866-76. https://doi.org/10.1056/NEJMra1915327.
[11] Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. (2008). Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28(3):226-36. https://doi.org/10.1111/j.1468-2982.2007.01497.x.
[12] Pellesi L, Al-Karagholi MA, De Icco R, Coskun H, Elbahi FA, Lopez-Lopez C, et al. (2021). Effect of Vasoactive Intestinal Polypeptide on Development of Migraine Headaches: A Randomized Clinical Trial. JAMA Netw Open. 2021;4(8):e2118543. https://doi.org/10.1001/jamanetworkopen.2021.18543.
[13] Ramachandran R. (2018). Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40(3):301-14. https://doi.org/10.1007/s00281-018-0676-y.
[14] Stankewitz A, Keidel L, Rehm M, Irving S, Kaczmarz S, Preibisch C, et al. (2021). Migraine attacks as a result of hypothalamic loss of control. Neuroimage Clin. 2021;32:102784. https://doi.org/10.1016/j.nicl.2021.102784.
[15] Gollion C, De Icco R, Dodick DW, Ashina H. (2022). The premonitory phase of migraine is due to hypothalamic dysfunction: revisiting the evidence. J Headache Pain. 2022;23(1):158. https://doi.org/10.1186/s10194-022-01518-5.
[16] Malu OO, Bailey J, Hawks MK. (2022). Cluster Headache: Rapid Evidence Review. Am Fam Physician. 2022;105(1):24-32.
[17] Steel SJ, Robertson CE, Whealy MA. (2021). Current Understanding of the Pathophysiology and Approach to Tension-Type Headache. Curr Neurol Neurosci Rep. 2021;21(10):56. https://doi.org/10.1007/s11910-021-01138-7.
[18] Edvinsson L, Goadsby PJ. (2019). Discovery of CGRP in relation to migraine. Cephalalgia. 2019;39(3):331-2. https://doi.org/10.1177/0333102418779544.
[19] Bedrin K, Shah T, Vaidya S, Ailani J. (2024). CGRP Modulating Therapies: An Update. Curr Neurol Neurosci Rep. 2024;24(9):453-9. https://doi.org/10.1007/s11910-024-01363-w.
[20] Gárate G, Pascual J, Pascual-Mato M, Madera J, Martín MM, González-Quintanilla V. (2024). Untangling the mess of CGRP levels as a migraine biomarker: an in-depth literature review and analysis of our experimental experience. J Headache Pain. 2024;25(1):69. https://doi.org/10.1186/s10194-024-01769-4.
[21] Kamm K. (2022). CGRP and Migraine: What Have We Learned From Measuring CGRP in Migraine Patients So Far? Front Neurol. 2022;13:930383. https://doi.org/10.3389/fneur.2022.930383.
[22] Kamm K, Straube A, Ruscheweyh R. (2019). Calcitonin gene-related peptide levels in tear fluid are elevated in migraine patients compared to healthy controls. Cephalalgia. 2019;39(12):1535-43. https://doi.org/10.1177/0333102419856640.
[23] Alpuente A, Gallardo VJ, Asskour L, Caronna E, Torres-Ferrus M, Pozo-Rosich P. (2022). Salivary CGRP can monitor the different migraine phases: CGRP (in)dependent attacks. Cephalalgia. 2022;42(3):186-96. https://doi.org/10.1177/03331024211040467.
[24] Waliszewska-Prosół M, Vuralli D, Martelletti P. (2023). What to do with non-responders to CGRP(r) monoclonal antibodies: switch to another or move to gepants? J Headache Pain. 2023;24(1):163. https://doi.org/10.1186/s10194-023-01698-8.
[25] An YC, Hung KS, Liang CS, Tsai CK, Tsai CL, Chen SJ, et al. (2024). Genetic variants associated with response to anti-CGRP monoclonal antibody therapy in a chronic migraine Han Chinese population. J Headache Pain. 2024;25(1):149. https://doi.org/10.1186/s10194-024-01850-y.
[26] Pellesi L, Ashina M, Martelletti P. (2024). Targeting the PACAP-38 pathway is an emerging therapeutic strategy for migraine prevention. Expert Opin Emerg Drugs. 2024;29(1):57-64. https://doi.org/10.1080/14728214.2024.2317778.
[27] Pellesi L, De Icco R, Al-Karagholi MA, Ashina M. (2020). Reducing Episodic Cluster Headaches: Focus on Galcanezumab. J Pain Res. 2020;13:1591-9. https://doi.org/10.2147/JPR.S222604.
[28] Medrea I, Christie S, Tepper SJ, Thavorn K, Hutton B. (2022). Effects of acute and preventive therapies for episodic and chronic cluster headache: A scoping review of the literature. Headache. 2022;62(3):329-62. https://doi.org/10.1111/head.14284.
[29] Petersen AS, Lund N, Goadsby PJ, Belin AC, Wang SJ, Fronczek R, et al. (2024). Recent advances in diagnosing, managing, and understanding the pathophysiology of cluster headache. Lancet Neurol. 2024;23(7):712-24. https://doi.org/10.1016/S1474-4422(24)00143-1.
[30] Gupta R, Ahmed T, Banerjee B, Bhatia M. (2009). Plasma calcitonin gene-related peptide concentration is comparable to control group among migraineurs and tension type headache subjects during inter-ictal period. J Headache Pain. 2009;10(3):161-6. https://doi.org/10.1007/s10194-009-0110-x.
[31] Bach FW, Langemark M, Ekman R, Rehfeld JF, Schifter S, Olesen J. (1994). Effect of sulpiride or paroxetine on cerebrospinal fluid neuropeptide concentrations in patients with chronic tension-type headache. Neuropeptides. 1994;27(2):129-36. https://doi.org/10.1016/0143-4179(94)90053-1.
[32] Moller K, Zhang YZ, Håkanson R, Luts A, Sjölund B, Uddman R, et al. (1993). Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience. 1993;57(3):725-32. https://doi.org/10.1016/0306-4522(93)90018-b.
[33] Mulder H, Uddman R, Moller K, Zhang YZ, Ekblad E, Alumets J, et al. (1994). Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience. 1994;63(1):307-12. https://doi.org/10.1016/0306-4522(94)90025-6.
[34] Edvinsson L, Elsås T, Suzuki N, Shimizu T, Lee TJ. (2001). Origin and co-localization of nitric oxide synthase, CGRP, PACAP, and VIP in the cerebral circulation of the rat. Microsc Res Tech. 2001;53(3):221-8. https://doi.org/10.1002/jemt.1086.
[35] Ashina M, Phul R, Khodaie M, Löf E, Florea I. (2024). A Monoclonal Antibody to PACAP for Migraine Prevention. N Engl J Med. 2024;391(9):800-9. https://doi.org/10.1056/NEJMoa2314577.
[36] Ashina M, Doležil D, Bonner JH, Zhou L, Klatt J, Picard H, et al. (2021). A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention. Cephalalgia. 2021;41(1):33-44. https://doi.org/10.1177/0333102420970889.
[37] Guo S, Rasmussen RH, Hay-Schmidt A, Ashina M, Asuni AA, Jensen JM, et al. (2024). VPAC1 and VPAC2 receptors mediate tactile hindpaw hypersensitivity and carotid artery dilatation induced by PACAP38 in a migraine relevant mouse model. J Headache Pain. 2024;25(1):126. https://doi.org/10.1186/s10194-024-01830-2.
[38] Pedersen SH, la Cour SH, Calloe K, Hauser F, Olesen J, Klaerke DA, et al. (2019). PACAP-38 and PACAP(6-38) Degranulate Rat Meningeal Mast Cells via the Orphan MrgB3-Receptor. Front Cell Neurosci. 2019;13:114. https://doi.org/10.3389/fncel.2019.00114.
[39] Staton PC, Hatcher JP, Walker DJ, Morrison AD, Shapland EM, Hughes JP, et al. (2008). The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain. 2008;139(1):225-36. https://doi.org/10.1016/j.pain.2008.04.006.
[40] Han X, Dong Z, Hou L, Wan D, Chen M, Tang W, et al. (2015). Interictal plasma pituitary adenylate cyclase-activating polypeptide levels are decreased in migraineurs but remain unchanged in patients with tension-type headache. Clin Chim Acta. 2015;450:151-4. https://doi.org/10.1016/j.cca.2015.08.017.
[41] Tuka B, Szabó N, Tóth E, Kincses ZT, Párdutz Á, Szok D, et al. (2016). Release of PACAP-38 in episodic cluster headache patients—an exploratory study. J Headache Pain. 2016;17(1):69. https://doi.org/10.1186/s10194-016-0660-7.
[42] Vollesen ALH, Snoer A, Chaudhry B, Petersen AS, Hagedorn A, Hoffmann J, et al. (2020). The effect of pituitary adenylate cyclase-activating peptide-38 and vasoactive intestinal peptide in cluster headache. Cephalalgia. 2020;40(13):1474-88. https://doi.org/10.1177/0333102420940689.
[43] Pellesi L, Chaudhry BA, Vollesen ALH, Snoer AH, Baumann K, Skov PS, et al. (2022). PACAP38- and VIP-induced cluster headache attacks are not associated with changes of plasma CGRP or markers of mast cell activation. Cephalalgia. 2022;42(8):687-95. https://doi.org/10.1177/03331024211056248.
[44] Deligianni C, Pellesi L, Chaudhry BA, Haulund Vollesen AL, Snoer AH, Hannibal J, et al. (2023). Plasma levels of VIP are not elevated during PACAP- and VIP-induced cluster headache attacks: an exploratory study. Front Neurol. 2023;14:1135246. https://doi.org/10.3389/fneur.2023.1135246.
[45] Tanaka M, Szabó Á, Körtési T, Szok D, Tajti J, Vécsei L. (2023). From CGRP to PACAP, VIP, and Beyond: Unraveling the Next Chapters in Migraine Treatment. Cells. 2023;12(22):2649. https://doi.org/10.3390/cells12222649.
 Figures
Figures References
References Peer
Peer Information
InformationFigure 1. Neurovascular Mechanisms in Primary Headaches at the Trigeminovascular Interface. CH: cluster headache. CGRP: calcitonin gene-related peptide. PACAP: pituitary adenylate cyclase-activating polypeptide. TTH: tension-type headache.

[1] Waliszewska-Prosół M, Montisano DA, Antolak M, Bighiani F, Cammarota F, Cetta I, et al. (2024). The impact of primary headaches on disability outcomes: a literature review and meta-analysis to inform future iterations of the Global Burden of Disease study. J Headache Pain. 2024;25(1):27. https://doi.org/10.1186/s10194-024-01735-0.
[2] Moskowitz MA. (2007). Pathophysiology of headache—past and present. Headache. 2007;47 Suppl 1:S58-63. https://doi.org/10.1111/j.1526-4610.2007.00678.x.
[3] Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. (2019). Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol. 2019;18(8):795-804. https://doi.org/10.1016/S1474-4422(19)30185-1.
[4] Guo S, Jansen-Olesen I, Olesen J, Christensen SL. (2023). Role of PACAP in migraine: An alternative to CGRP? Neurobiol Dis. 2023;176:105946. https://doi.org/10.1016/j.nbd.2022.105946.
[5] Kuburas A, Russo AF. (2023). Shared and independent roles of CGRP and PACAP in migraine pathophysiology. J Headache Pain. 2023;24(1):34. https://doi.org/10.1186/s10194-023-01569-2.
[6] Jacobs B, Dussor G. (2016). Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience. 2016;338:130-44. https://doi.org/10.1016/j.neuroscience.2016.06.012.
[7] Ray B, Wolff H. (1940). Experimental studies on headache. Pain sensitive structures of the head and their significance in headache. Arch Surg. 1940;41(4):813-56. https://doi.org/10.1001/archsurg.1940.01210040002001.
[8] Tunis MM, Wolff HG. (1953). Studies on headache; long-term observations of the reactivity of the cranial arteries in subjects with vascular headache of the migraine type. AMA Arch Neurol Psychiatry. 1953;70(5):551-7. https://doi.org/10.1001/archneurpsyc.1953.02320350003001.
[9] Goadsby PJ. (2009). The vascular theory of migraine—a great story wrecked by the facts. Brain. 2009;132(Pt 1):6-7. https://doi.org/10.1093/brain/awn321.
[10] Ashina M. (2020). Migraine. N Engl J Med. 2020;383(19):1866-76. https://doi.org/10.1056/NEJMra1915327.
[11] Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. (2008). Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28(3):226-36. https://doi.org/10.1111/j.1468-2982.2007.01497.x.
[12] Pellesi L, Al-Karagholi MA, De Icco R, Coskun H, Elbahi FA, Lopez-Lopez C, et al. (2021). Effect of Vasoactive Intestinal Polypeptide on Development of Migraine Headaches: A Randomized Clinical Trial. JAMA Netw Open. 2021;4(8):e2118543. https://doi.org/10.1001/jamanetworkopen.2021.18543.
[13] Ramachandran R. (2018). Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40(3):301-14. https://doi.org/10.1007/s00281-018-0676-y.
[14] Stankewitz A, Keidel L, Rehm M, Irving S, Kaczmarz S, Preibisch C, et al. (2021). Migraine attacks as a result of hypothalamic loss of control. Neuroimage Clin. 2021;32:102784. https://doi.org/10.1016/j.nicl.2021.102784.
[15] Gollion C, De Icco R, Dodick DW, Ashina H. (2022). The premonitory phase of migraine is due to hypothalamic dysfunction: revisiting the evidence. J Headache Pain. 2022;23(1):158. https://doi.org/10.1186/s10194-022-01518-5.
[16] Malu OO, Bailey J, Hawks MK. (2022). Cluster Headache: Rapid Evidence Review. Am Fam Physician. 2022;105(1):24-32.
[17] Steel SJ, Robertson CE, Whealy MA. (2021). Current Understanding of the Pathophysiology and Approach to Tension-Type Headache. Curr Neurol Neurosci Rep. 2021;21(10):56. https://doi.org/10.1007/s11910-021-01138-7.
[18] Edvinsson L, Goadsby PJ. (2019). Discovery of CGRP in relation to migraine. Cephalalgia. 2019;39(3):331-2. https://doi.org/10.1177/0333102418779544.
[19] Bedrin K, Shah T, Vaidya S, Ailani J. (2024). CGRP Modulating Therapies: An Update. Curr Neurol Neurosci Rep. 2024;24(9):453-9. https://doi.org/10.1007/s11910-024-01363-w.
[20] Gárate G, Pascual J, Pascual-Mato M, Madera J, Martín MM, González-Quintanilla V. (2024). Untangling the mess of CGRP levels as a migraine biomarker: an in-depth literature review and analysis of our experimental experience. J Headache Pain. 2024;25(1):69. https://doi.org/10.1186/s10194-024-01769-4.
[21] Kamm K. (2022). CGRP and Migraine: What Have We Learned From Measuring CGRP in Migraine Patients So Far? Front Neurol. 2022;13:930383. https://doi.org/10.3389/fneur.2022.930383.
[22] Kamm K, Straube A, Ruscheweyh R. (2019). Calcitonin gene-related peptide levels in tear fluid are elevated in migraine patients compared to healthy controls. Cephalalgia. 2019;39(12):1535-43. https://doi.org/10.1177/0333102419856640.
[23] Alpuente A, Gallardo VJ, Asskour L, Caronna E, Torres-Ferrus M, Pozo-Rosich P. (2022). Salivary CGRP can monitor the different migraine phases: CGRP (in)dependent attacks. Cephalalgia. 2022;42(3):186-96. https://doi.org/10.1177/03331024211040467.
[24] Waliszewska-Prosół M, Vuralli D, Martelletti P. (2023). What to do with non-responders to CGRP(r) monoclonal antibodies: switch to another or move to gepants? J Headache Pain. 2023;24(1):163. https://doi.org/10.1186/s10194-023-01698-8.
[25] An YC, Hung KS, Liang CS, Tsai CK, Tsai CL, Chen SJ, et al. (2024). Genetic variants associated with response to anti-CGRP monoclonal antibody therapy in a chronic migraine Han Chinese population. J Headache Pain. 2024;25(1):149. https://doi.org/10.1186/s10194-024-01850-y.
[26] Pellesi L, Ashina M, Martelletti P. (2024). Targeting the PACAP-38 pathway is an emerging therapeutic strategy for migraine prevention. Expert Opin Emerg Drugs. 2024;29(1):57-64. https://doi.org/10.1080/14728214.2024.2317778.
[27] Pellesi L, De Icco R, Al-Karagholi MA, Ashina M. (2020). Reducing Episodic Cluster Headaches: Focus on Galcanezumab. J Pain Res. 2020;13:1591-9. https://doi.org/10.2147/JPR.S222604.
[28] Medrea I, Christie S, Tepper SJ, Thavorn K, Hutton B. (2022). Effects of acute and preventive therapies for episodic and chronic cluster headache: A scoping review of the literature. Headache. 2022;62(3):329-62. https://doi.org/10.1111/head.14284.
[29] Petersen AS, Lund N, Goadsby PJ, Belin AC, Wang SJ, Fronczek R, et al. (2024). Recent advances in diagnosing, managing, and understanding the pathophysiology of cluster headache. Lancet Neurol. 2024;23(7):712-24. https://doi.org/10.1016/S1474-4422(24)00143-1.
[30] Gupta R, Ahmed T, Banerjee B, Bhatia M. (2009). Plasma calcitonin gene-related peptide concentration is comparable to control group among migraineurs and tension type headache subjects during inter-ictal period. J Headache Pain. 2009;10(3):161-6. https://doi.org/10.1007/s10194-009-0110-x.
[31] Bach FW, Langemark M, Ekman R, Rehfeld JF, Schifter S, Olesen J. (1994). Effect of sulpiride or paroxetine on cerebrospinal fluid neuropeptide concentrations in patients with chronic tension-type headache. Neuropeptides. 1994;27(2):129-36. https://doi.org/10.1016/0143-4179(94)90053-1.
[32] Moller K, Zhang YZ, Håkanson R, Luts A, Sjölund B, Uddman R, et al. (1993). Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience. 1993;57(3):725-32. https://doi.org/10.1016/0306-4522(93)90018-b.
[33] Mulder H, Uddman R, Moller K, Zhang YZ, Ekblad E, Alumets J, et al. (1994). Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience. 1994;63(1):307-12. https://doi.org/10.1016/0306-4522(94)90025-6.
[34] Edvinsson L, Elsås T, Suzuki N, Shimizu T, Lee TJ. (2001). Origin and co-localization of nitric oxide synthase, CGRP, PACAP, and VIP in the cerebral circulation of the rat. Microsc Res Tech. 2001;53(3):221-8. https://doi.org/10.1002/jemt.1086.
[35] Ashina M, Phul R, Khodaie M, Löf E, Florea I. (2024). A Monoclonal Antibody to PACAP for Migraine Prevention. N Engl J Med. 2024;391(9):800-9. https://doi.org/10.1056/NEJMoa2314577.
[36] Ashina M, Doležil D, Bonner JH, Zhou L, Klatt J, Picard H, et al. (2021). A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention. Cephalalgia. 2021;41(1):33-44. https://doi.org/10.1177/0333102420970889.
[37] Guo S, Rasmussen RH, Hay-Schmidt A, Ashina M, Asuni AA, Jensen JM, et al. (2024). VPAC1 and VPAC2 receptors mediate tactile hindpaw hypersensitivity and carotid artery dilatation induced by PACAP38 in a migraine relevant mouse model. J Headache Pain. 2024;25(1):126. https://doi.org/10.1186/s10194-024-01830-2.
[38] Pedersen SH, la Cour SH, Calloe K, Hauser F, Olesen J, Klaerke DA, et al. (2019). PACAP-38 and PACAP(6-38) Degranulate Rat Meningeal Mast Cells via the Orphan MrgB3-Receptor. Front Cell Neurosci. 2019;13:114. https://doi.org/10.3389/fncel.2019.00114.
[39] Staton PC, Hatcher JP, Walker DJ, Morrison AD, Shapland EM, Hughes JP, et al. (2008). The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain. 2008;139(1):225-36. https://doi.org/10.1016/j.pain.2008.04.006.
[40] Han X, Dong Z, Hou L, Wan D, Chen M, Tang W, et al. (2015). Interictal plasma pituitary adenylate cyclase-activating polypeptide levels are decreased in migraineurs but remain unchanged in patients with tension-type headache. Clin Chim Acta. 2015;450:151-4. https://doi.org/10.1016/j.cca.2015.08.017.
[41] Tuka B, Szabó N, Tóth E, Kincses ZT, Párdutz Á, Szok D, et al. (2016). Release of PACAP-38 in episodic cluster headache patients—an exploratory study. J Headache Pain. 2016;17(1):69. https://doi.org/10.1186/s10194-016-0660-7.
[42] Vollesen ALH, Snoer A, Chaudhry B, Petersen AS, Hagedorn A, Hoffmann J, et al. (2020). The effect of pituitary adenylate cyclase-activating peptide-38 and vasoactive intestinal peptide in cluster headache. Cephalalgia. 2020;40(13):1474-88. https://doi.org/10.1177/0333102420940689.
[43] Pellesi L, Chaudhry BA, Vollesen ALH, Snoer AH, Baumann K, Skov PS, et al. (2022). PACAP38- and VIP-induced cluster headache attacks are not associated with changes of plasma CGRP or markers of mast cell activation. Cephalalgia. 2022;42(8):687-95. https://doi.org/10.1177/03331024211056248.
[44] Deligianni C, Pellesi L, Chaudhry BA, Haulund Vollesen AL, Snoer AH, Hannibal J, et al. (2023). Plasma levels of VIP are not elevated during PACAP- and VIP-induced cluster headache attacks: an exploratory study. Front Neurol. 2023;14:1135246. https://doi.org/10.3389/fneur.2023.1135246.
[45] Tanaka M, Szabó Á, Körtési T, Szok D, Tajti J, Vécsei L. (2023). From CGRP to PACAP, VIP, and Beyond: Unraveling the Next Chapters in Migraine Treatment. Cells. 2023;12(22):2649. https://doi.org/10.3390/cells12222649.
Peer-review Terminology
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published:
Review reports
Reviewer identities if reviewer opts in
Author/reviewer communication
Details
© 2025 The Author(s). Brain Conflux published by Life Conflux Press Limited on behalf of Conflux Science.
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Publication History
Received 2025-04-29
Accepted 2025-05-22
Published 2025-06-01


