Abstract
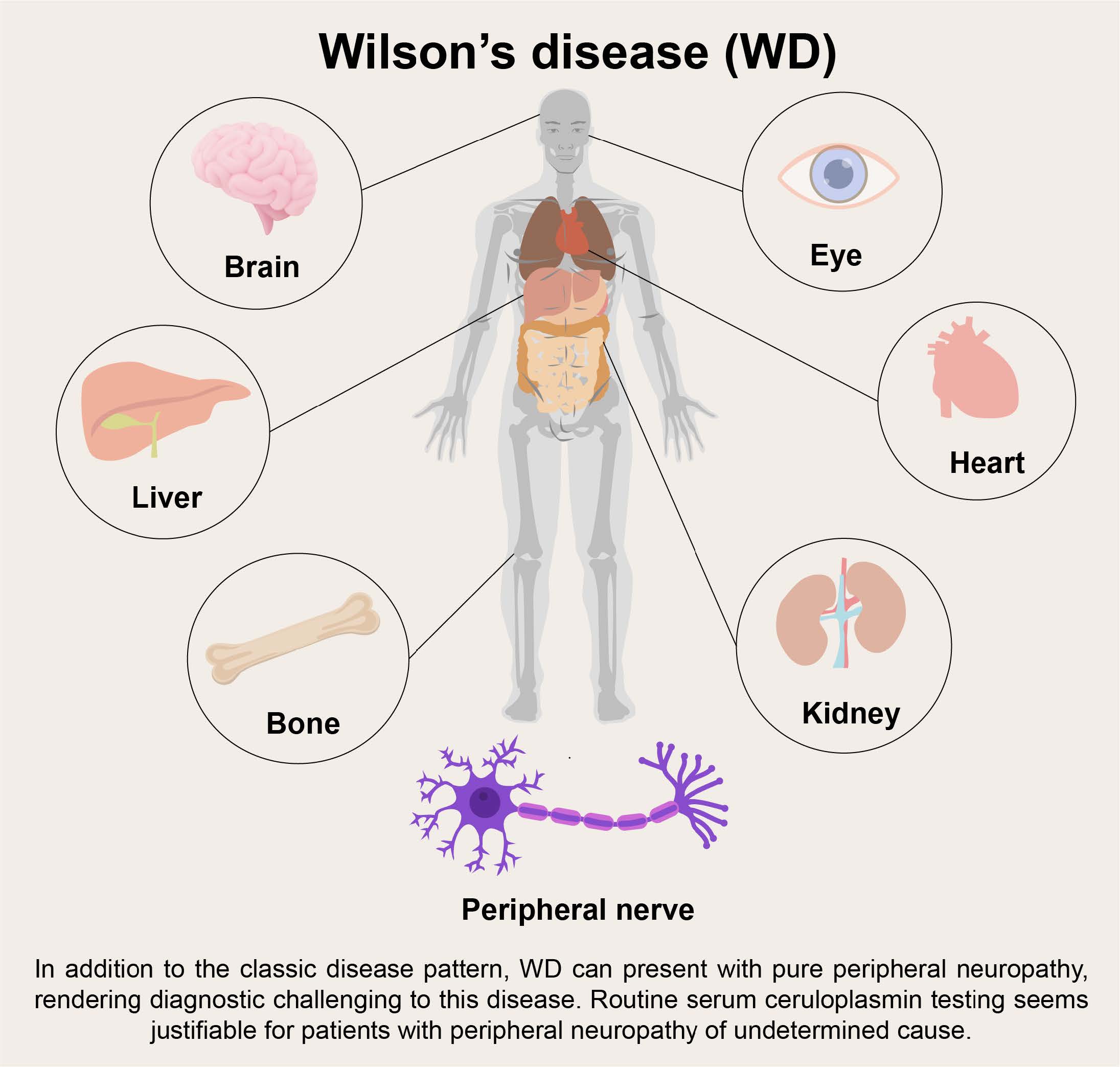
Background:Wilson's disease (WD) is an inherited autosomal recessive disease affecting copper metabolism in the body. Nearly all neurological symptoms are attributed to the central nervous system, while peripheral neuropathy is rare.
Case presentation:A 48-year-old male presented with progressive muscle weakness and atrophy in distal extremities for 7 months. Pure distal motor axonal neuropathy was confirmed by electromyography. Extremely low serum ceruloplasmin level was detected accidentally and gene screening of ATP7B found two known compound heterozygous mutations (c.3859G>A p.(Gly1287Ser), c3155C>T p.(Pro1052Leu)). However, the patient had no typical symptoms or signs of WD. Brain MRI was unremarkable. B ultrasound only revealed mild liver steatosis. He responded well to penicillamine treatment. Electromyography showed nearly normal results 1 year later.
Conclusion:WD can present with pure peripheral neuropathy, rendering diagnostic challenging to this disease. Routine serum ceruloplasmin testing seems justifiable for patients with peripheral neuropathy of undetermined cause.
Keywords:Wilson's disease; peripheral neuropathy; hypoceruloplasminemia;
Background
Wilson’s disease (WD) is an inherited autosomal recessive disease affecting copper metabolism in the body caused by mutations in the ATP7B gene. Most WD patients show symptoms at <40 years of age [1] . Excessive copper accumulating in the central nervous system (CNS) leads to symptoms such as tremor, parkinsonism, dystonia, chorea, myoclonus, and psychiatric or psychological problems [2] . Copper overloading in the liver causes cirrhosis, which is found in most patients by the second decade of life, and steatosis is usually an early sign [1] . Kayser-Fleischer (K-F) ring is a specific sign presenting in 85%-100% of neurological cases, 33-86% of hepatic cases, and 0-59% in asymptomatic patients [3] . However, involvement of the peripheral nervous system is uncommon for WD. Although subtle peripheral nerve changes can be observed pathologically [4,5] or detected by electromyography (EMG) [6] , clinical manifestations are nearly imperceptible. Herein, we report a peculiar late-onset WD case who presented with peripheral neuropathy exclusively and responded to penicillamine treatment. No examinations suggested WD other than low serum ceruloplasmin level.
Case presentation
A previously well 48-year-old Chinese male was admitted in September 2015 after he suffered from weakness and atrophy in distal extremities without sensation complaints for 7 months. No infection, intoxication, or diabetes could be traced. Before admission, he had been taking corticosteroids (taping from 60mg daily) for 2 months but it was of no avail. Physical examination highlighted distal extremities weakness (MRC (Medical Research Council) grade 4+), decreased muscle bulks in hands, shanks, and feet (Figure 1), absent tendon reflexes, and unsteady tandem gait. Sensory examination was unremarkable.EMG demonstrated positive sharp waves and fibrillation potentials in the biceps brachii, first dorsal interosseous, flexor carpi radialis, extensor digitorum brevis, popliteal muscle, and tibialis anterior muscles with giant motor unit potentials. A nerve conduction study showed a greatly decreased amplitude of compound motor action potential (CMAP) with normal F-wave latency, distal motor latency, and motor nerve conduction velocity (MNCV) in most motor nerves (Table 1). Sensory nerves were not affected. Most laboratory tests relevant to peripheral neuropathy were unremarkable, including blood glucose, liver function, vitamin B12, vitamin B1, foliate acid levels, monoclonal protein, RF, ANA, dsDNA, ANCA, and anti-ganglioside antibodies. Hemoglobin was 118g/l (reference range:120-160g/l). Serum ceruloplasmin as a risk factor screening showed less than 0.02g/l (reference range:0.2-0.4g/l) and serum copper level was 1.7umol/l (reference range:11-24umol/l),and then 24-hour urinary copper excretion level was <0.10umol/24h . Cerebrospinal fluid analysis revealed an elevated protein level of 1.4 g/l (reference range:0.15-0.45 g/l) but a normal cell count.
Further investigations for WD were as follows: abdominal B ultrasound which detected mild liver steatosis and splenomegaly (131*43mm), brain MRI revealing normal results, and slit lamp examination showing no K-F rings. Gene screening of ATP7A and ATP7B was performed. Two previously reported mutations, c.3859G>A p.(Gly1287Ser) and c3155C>T p.(Pro1052Leu), were detected in ATP7B. No mutations were found in ATP7A. His family members were requested to mail their blood samples for gene screening. The pedigree tree showed his father carried the mutation of c.3859G>A p.(Gly1287Ser), while his mother was deceased. His elder sister and younger brother carried neither of the mutations and his elder brother carried both. None of his family members but his elder brother reported mild walking unsteadiness,which did not affect his life, and refused further medical examination. However, he refused to visit our clinic. According to the gene screening result, WD was diagnosed. The patient was started on 125mg penicillamine TID. In December 2015, he reported improvement in his strength. In October 2016, he could walk without difficulty and his muscle bulks increased (Figure 1). Muscle strength was normal (MRC grade 5). Hemoglobin elevated to 165g/l and fatty liver disappeared on ultrasound. EMG showed no positive sharp waves. A nerve conduction study showed normal CMAPs in most motor nerves (Table 1).
Figure 1: Atrophy of intrinsic muscle of hands (A) and tibialis anterior muscle (C) in September 2015. Muscle bulks increased after 1-year-long penicillamine treatment (B, D).
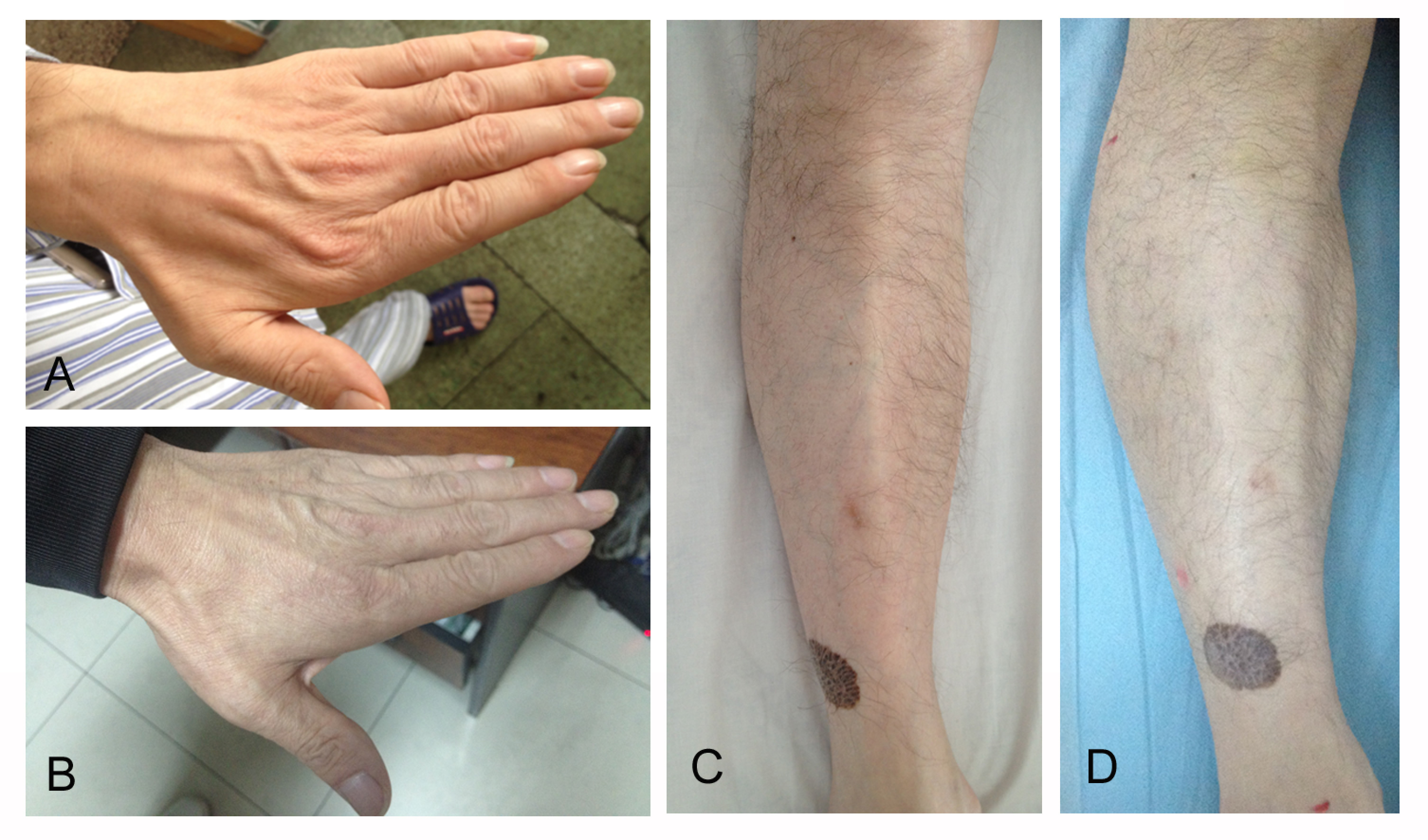
Table 1:Findings of nerve conduction studies in July 2015 and October 2016. The first nerve conduction study was performed in July 2015 and the second one was in October 2016. Abbreviations: CMAP: compound muscle action potential, MNCV: motor nerve conduct velocity, ADM: abductor digiti minimi, APB: abductor pollicis brevis, AH: abductor hallucis, EDB: extensor digitorum brevis.
|
Latency(ms) |
CMAP(mv) |
MNCV(m/s) |
F-wave latency(ms) |
|||||||||
|
|
1st |
2nd |
Cutoff value |
1st |
2nd |
Cutoff value |
1st |
2nd |
Cutoff value |
1st |
2nd |
Cutoff value |
|
Ulnar nerve |
|
|
|
|
|
≥5.5 |
|
|
≥50 |
|
|
≤31.2 |
|
Wrist-ADM |
2.69 |
3.2 |
≤3.1 |
2.4 |
9.8 |
|
|
|
|
28 |
28.8 |
|
|
Below elbow-wrist |
6.26 |
7.33 |
|
2.3 |
9.0 |
|
61.1 |
53.3 |
|
|
|
|
|
Below-above elbow |
8.40 |
9.38 |
|
2.2 |
9.3 |
|
51.4 |
58 |
|
|
|
|
|
Media nerve |
|
|
|
|
|
≥4.8 |
|
|
≥50 |
|
|
≤30 |
|
Wrist-APB |
3.71 |
4.44 |
≤4.2 |
1.21 |
8.2 |
|
|
|
|
29 |
27.5 |
|
|
Axillary-wrist |
7.92 |
8.31 |
|
0.91 |
7.6 |
|
54.6 |
56.8 |
|
|
|
|
|
Tibia nerve |
|
|
|
|
|
≥5.0 |
|
|
|
|
|
|
|
Ankle-AH |
4.19 |
4.59 |
≤5.8 |
1.69 |
2.5 |
|
|
|
≥39.4 |
|
|
≤59.2 |
|
popliteal-ankle |
12.9 |
12.5 |
|
1.52 |
1.95 |
|
45.1 |
47.4 |
|
50.4 |
51.1 |
|
|
Peroneal nerve |
|
|
|
|
|
≥2.3 |
|
|
≥39.8 |
|
|
≤59 |
|
Ankle-EDB |
3.88 |
3.90 |
≤4.6 |
3.1 |
4.3 |
|
|
|
|
47.4 |
48.9 |
|
|
Below knee-ankle |
10.0 |
10.5 |
|
3.0 |
3.8 |
|
43.3 |
45 |
|
|
|
|
|
Above knee-below knee |
11.9 |
13 |
|
2.9 |
3.7 |
|
47.4 |
41.2 |
|
|
|
|
Discussion and conclusion
Several studies demonstrate subclinical peripheral nerve changes in WD. Bowles RP, et al. verified small areas of demyelination in peripheral nerves by autopsy [7] . Sturniolo GC, et al. observed reduced nerve fiber density, number of branching number of beading, and more fiber tortuosity in WD patients’ cornea [4] . In Gondim Fde A’s study, water-induced skin wrinkling test which tests autonomic/sensory small fiber function was positive in two patients [8] . Pathogenesis of peripheral fiber damage in WD is largely unknown. Copper toxicity is probably secondary to the formation of reactive oxygen species. Overproduction of oxidizing radicals leads to the impairment of essential molecules, such as lipids, proteins, and DNA. Mitochondria damage by oxidative stress may suppress neuron function [4] . Copper is involved in myelination, and this may be the cause of the peripheral nerve injury in the patient.
Despite the pathological and electrophysical evidence of peripheral nerve damage, symptomatic neuropathy is extremely rare in WD patients if those caused by excessive de-copper treatment [9,10] are not counted. Only Jung KH, et al. reported a 17-year-old male patient presented with concrete sensorimotor peripheral neuropathy initially [11] . WD was not considered until movement disorders developed and typical signs of WD, such as KF ring, cirrhosis, and hypoceruloplasminemia were found [11] . Compared with that case, the clinical picture of our patient is far less typical. The onset age of 48 in this case is very late. Only 3.8% of patients were symptomatic after 40 years of age and 1.2% became symptomatic after 48 [12] . Our patient only had liver steatosis and hypoceruloplasminemia. Liver steatosis is never specific for most liver diseases. So, hypoceruloplasminemia seems to be the only breakthrough. Although hypoceruloplasminemia not always suggests WD, extremely low ceruloplasmin level (<50 mg/L or <5 mg/dL) should be taken as strong evidence for the diagnosis of WD [1] . The diagnosis was confirmed by gene screening of ATP7B. The mutations, c.3859G>A p.(Gly1287Ser) and c.3155C>T p.(Pro1052Leu), were previously reported [13,14] . In copper-related diseases, ATP7A-related polyneuropathy has been reported, but serum ceruloplasmin is usually normal in this condition [15] . In our case, gene screening of ATP7A is normal. We speculated that the copper metabolic pathway may be individualized. Although ceruloplasmin was extremely low in our patient, urine ketone did not increase, and excess copper may be deposited more on peripheral nerves.
In conclusion, we present a case of WD where peripheral neuropathy appeared as the only symptom. WD is a potentially treatable disease, so recognizing this rare form of WD is of value. Serum ceruloplasmin level which is not routinely included in the neuropathy panel should be tested in patients with polyneuropathy of undetermined causes.
Acknowledgements
We thank Dr Qingdong Zhu for interpreting EMG results.
Author contributions
W.G.: patient management, initial manuscript preparation; F.Q.L.: initial manuscript preparation; Y.M.S.: initial manuscript preparation; B.A.K.: critical review; D.Q.Z: interpretation of EMG results and critical review. Y.Z.: patient management, critical review; final approval of the manuscript to be submitted. All authors have read and approved the manuscript of “Wilson’s disease presenting with Polyneuropathy: a case report”. W.G., F.Q.L., and Y.M.S. contributed equally to the work.
Ethics approval and consent to participate
The study is approved by the ethics committee of Huashan Hospital.
Funding information
None
Competing Interests
The authors declare that they have no existing or potential commercial or financial relationships that could create a conflict of interest at the time of conducting this study.
Data Availability
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
References
[1] Roberts EA, Schilsky ML, American Association for Study of Liver D. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47(6):2089-2111.
[2] Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson's disease. Lancet. 2007;369(9559):397-408.
[3] Mak CM, Lam CW. Diagnosis of Wilson's disease: a comprehensive review. Crit Rev Clin Lab Sci. 2008;45(3):263-290.
[4] Sturniolo GC, Lazzarini D, Bartolo O, et al. Small fiber peripheral neuropathy in Wilson disease: an in vivo documentation by corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2015;56(2):1390-1395.
[5] Miyakawa T, Murayama E, Sumiyoshi S, Deshimaru M, Miyakawa K. A biopsy case of Wilson's disease. Pathological changes in peripheral nerves. Acta Neuropathol. 1973;24(2):174-177.
[6] Leven B, Fasshauer K. [Lesions of the peripheral nerves in Wilson's disease. Electrodiagnostic findings (author's transl)]. Fortschr Neurol Psychiatr Grenzgeb. 1978;46(4):202-206.
[7] Bowles RP. An unusual case of Wilson's disease. AMA Arch Intern Med. 1957;99(1):147-150.
[8] Gondim Fde A, Araujo DF, Oliveira IS, Vale OC. Small fiber dysfunction in patients with Wilson's disease. Arq Neuropsiquiatr. 2014;72(8):592-595.
[9] Cortese A, Zangaglia R, Lozza A, Piccolo G, Pacchetti C. Copper deficiency in Wilson's disease: peripheral neuropathy and myelodysplastic syndrome complicating zinc treatment. Mov Disord. 2011;26(7):1361-1362.
[10] Foubert-Samier A, Kazadi A, Rouanet M, et al. Axonal sensory motor neuropathy in copper-deficient Wilson's disease. Muscle Nerve. 2009;40(2):294-296.
[11] Jung KH, Ahn TB, Jeon BS. Wilson disease with an initial manifestation of polyneuropathy. Archives of neurology. 2005;62(10):1628-1631.
[12] Ferenci P, Czlonkowska A, Merle U, et al. Late-onset Wilson's disease. Gastroenterology. 2007;132(4):1294-1298.
[13] Curtis D, Durkie M, Balac P, et al. A study of Wilson disease mutations in Britain. Hum Mutat. 1999;14(4):304-311.
[14] Cox DW, Prat L, Walshe JM, Heathcote J, Gaffney D. Twenty-four novel mutations in Wilson disease patients of predominantly European ancestry. Hum Mutat. 2005;26(3):280.
[15] Bandmann O, Weiss KH, Kaler SG. Wilson's disease and other neurological copper disorders. Lancet Neurol. 2015;14(1):103-113.
 Figures
Figures References
References Peer
Peer Information
InformationFigure 1: Atrophy of intrinsic muscle of hands (A) and tibialis anterior muscle (C) in September 2015. Muscle bulks increased after 1-year-long penicillamine treatment (B, D).

[1] Roberts EA, Schilsky ML, American Association for Study of Liver D. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47(6):2089-2111.
[2] Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson's disease. Lancet. 2007;369(9559):397-408.
[3] Mak CM, Lam CW. Diagnosis of Wilson's disease: a comprehensive review. Crit Rev Clin Lab Sci. 2008;45(3):263-290.
[4] Sturniolo GC, Lazzarini D, Bartolo O, et al. Small fiber peripheral neuropathy in Wilson disease: an in vivo documentation by corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2015;56(2):1390-1395.
[5] Miyakawa T, Murayama E, Sumiyoshi S, Deshimaru M, Miyakawa K. A biopsy case of Wilson's disease. Pathological changes in peripheral nerves. Acta Neuropathol. 1973;24(2):174-177.
[6] Leven B, Fasshauer K. [Lesions of the peripheral nerves in Wilson's disease. Electrodiagnostic findings (author's transl)]. Fortschr Neurol Psychiatr Grenzgeb. 1978;46(4):202-206.
[7] Bowles RP. An unusual case of Wilson's disease. AMA Arch Intern Med. 1957;99(1):147-150.
[8] Gondim Fde A, Araujo DF, Oliveira IS, Vale OC. Small fiber dysfunction in patients with Wilson's disease. Arq Neuropsiquiatr. 2014;72(8):592-595.
[9] Cortese A, Zangaglia R, Lozza A, Piccolo G, Pacchetti C. Copper deficiency in Wilson's disease: peripheral neuropathy and myelodysplastic syndrome complicating zinc treatment. Mov Disord. 2011;26(7):1361-1362.
[10] Foubert-Samier A, Kazadi A, Rouanet M, et al. Axonal sensory motor neuropathy in copper-deficient Wilson's disease. Muscle Nerve. 2009;40(2):294-296.
[11] Jung KH, Ahn TB, Jeon BS. Wilson disease with an initial manifestation of polyneuropathy. Archives of neurology. 2005;62(10):1628-1631.
[12] Ferenci P, Czlonkowska A, Merle U, et al. Late-onset Wilson's disease. Gastroenterology. 2007;132(4):1294-1298.
[13] Curtis D, Durkie M, Balac P, et al. A study of Wilson disease mutations in Britain. Hum Mutat. 1999;14(4):304-311.
[14] Cox DW, Prat L, Walshe JM, Heathcote J, Gaffney D. Twenty-four novel mutations in Wilson disease patients of predominantly European ancestry. Hum Mutat. 2005;26(3):280.
[15] Bandmann O, Weiss KH, Kaler SG. Wilson's disease and other neurological copper disorders. Lancet Neurol. 2015;14(1):103-113.
Peer-review Terminology
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published:
Review reports
Reviewer identities if reviewer opts in
Author/reviewer communication
Details
© 2025 The Author(s). Brain Conflux published by Life Conflux Press Limited on behalf of Conflux Science.
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Publication History
Received 2025-01-25
Accepted 2025-03-27
Published 2025-03-30


